Home » News and events »
Promising target for liver disease treatments identified
An international clinical trial involving Birmingham Professor Phil Newsome identified a promising target for patients with non-alcoholic steatohepatitis (NASH), as reported in a paper published yesterday in Nature Communications. The research team demonstrated that an oral inhibitor of amine oxidase copper-containing 3 (AOC3), a protein involved in liver inflammation – which can lead to fibrosis, cirrhosis and liver cancer – successfully suppressed this protein, as well as reducing liver injury.
This study provides a clear rationale for AOC3 inhibition in the management of patients with metabolic dysfunction-associated steatotic liver disease.
– Professor Phil Newsome
The study, which tested an oral inhibitor produced by pharmaceutical company Boehringer Ingelheim, builds on the University of Birmingham’s long-standing work on AOC3 inhibition led by David Adams and Chris Weston, and validates this protein as a promising target to reduce liver inflammation, with the potential for subsequent beneficial effects on fibrosis in patients with NASH. The current standard of care for NASH is limited to lifestyle interventions, such as weight loss and exercise, which reduce inflammation and, indirectly, fibrosis. Building on this trial, pharmaceutical research company Pharmaxis will explore the potential to develop a safe and effective pharmacological treatment for NASH patients.
Study lead Phil Newsome, Professor of Hepatology at the University of Birmingham’s Institute of Immunology and Immunotherapy, Director of the Centre for Liver and Gastrointestinal Research and of the NIHR Birmingham Biomedical Research Centre, and Honorary Consultant Hepatologist at University Hospitals Birmingham NHS Foundation Trust, commented: “This study provides a clear rationale for AOC3 inhibition in the management of patients with metabolic dysfunction-associated steatotic liver disease.”
The trial
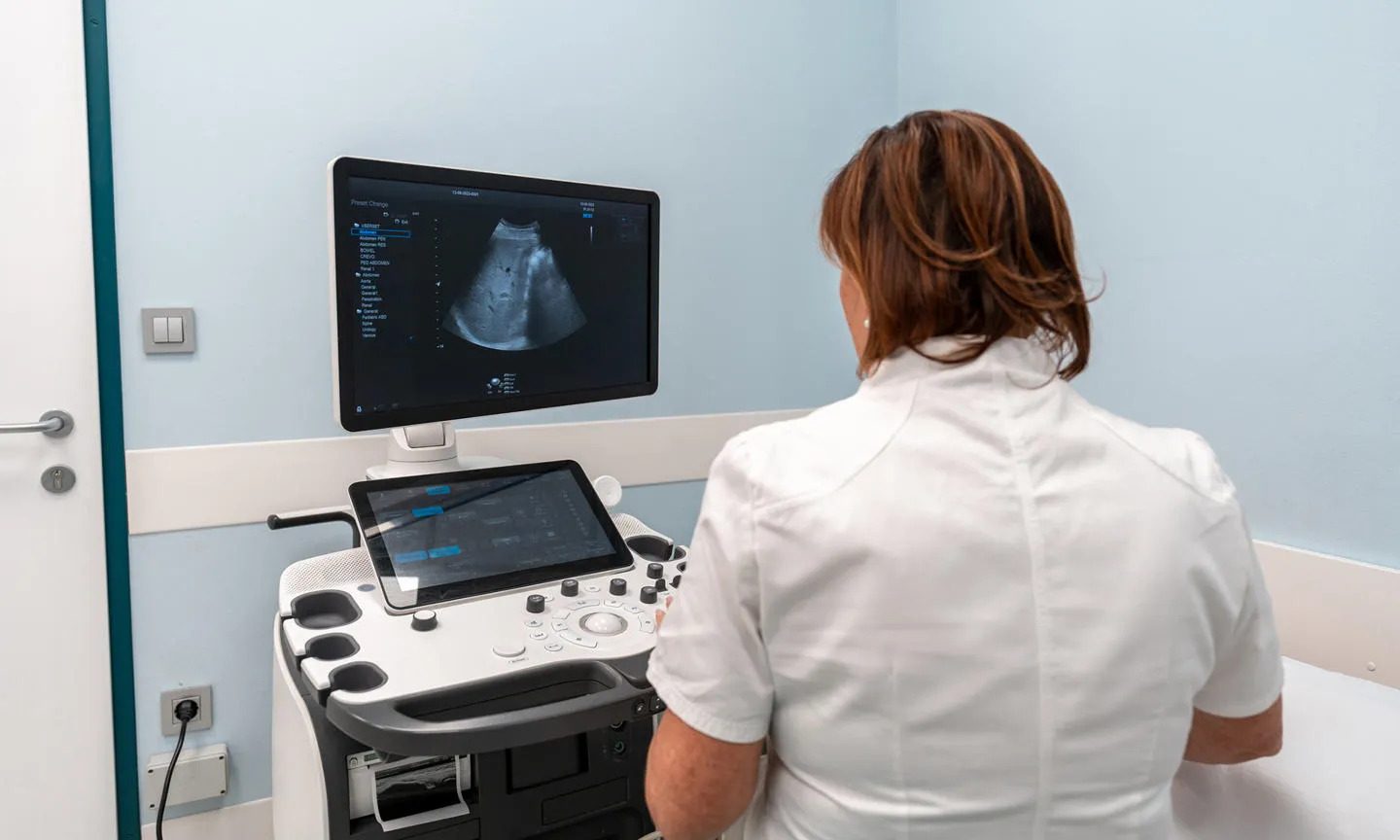
The Phase II trial, funded by Boehringer Ingelheim, aimed to assess the safety, tolerability and biochemical and physiological effects of different doses of oral AOC3 inhibitor BI 1467335 during a 12-week treatment period.
To do so, between 2017 and 2019 it enrolled 114 NASH patients from 44 centres across Europe and North America. Participants were randomly assigned to one of five treatment groups, who received at least one daily dose of either BI 1467335, in different dosages, or a placebo.
After 12 weeks of treatment, researchers observed that BI 1467335 strongly inhibited AOC3 in participants with NASH, with doses above a minimum threshold dependently reducing the levels of liver injury biomarkers.
The trial confirmed the importance of AOC3 as a target and will be taken forward by Pharmaxis.